Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Journal of Alloys and Compounds, 408-412, p.1274 - 1277, 2006/02
Times Cited Count:39 Percentile:84.47(Chemistry, Physical)For the development of advanced aqueous reprocessing system, it is one of the most important subjects to separate minor trivalent actinides (MA = Am and Cm). Recently, extraction selectivity for MA(III) over Ln(III) has been found in some extractants containing soft donor, such as S or N, ligands. Kolarik et al. reported that a new N-donor ligand 2,6-bis(5,6-dialkyl-1,2,4-triazine-3-yl)-pyridine (R-BTP) shows high selectivity for MA (III) over Ln(III). The novel silica-based extraction resins were prepared by impregnating some R-BTP molecules into a macroreticular styrene-divinylbenzene copolymer which is immobilized in porous silica particles with a mean diameter of 50  m. Separation of simulated high level liquid waste solution containing Ln(III) and trace amount of Am(III) was studied. Am(III) was mutually separated from Ln(III) through a packed column with R-BTP impregnating resin, very high decontamination factor (
m. Separation of simulated high level liquid waste solution containing Ln(III) and trace amount of Am(III) was studied. Am(III) was mutually separated from Ln(III) through a packed column with R-BTP impregnating resin, very high decontamination factor ( 10
10 ) for Am, and all the elements were recovered quantitatively.
) for Am, and all the elements were recovered quantitatively.
 -dimethylformamide
-dimethylformamideKim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Ikeda, Yasuhisa*
Journal of Alloys and Compounds, 408-412, p.1291 - 1295, 2006/02
Times Cited Count:10 Percentile:54.65(Chemistry, Physical)The electrochemical reactions of UO (
( -diketonato)
-diketonato) DMF, UO
DMF, UO (trop)
(trop) DMF and UO
DMF and UO (sap)(DMF)
(sap)(DMF) , (DMF = N,N-dimethylformamide,
, (DMF = N,N-dimethylformamide,  -diketonate = thenoyltrifluoroacetonate (ttfa), benzoyltrifluoroacetonate (btfa), and dibenzoylmethanate(dbm), trop = tropolonate, and sap = 2-salicylidenaminophenolate) complexes in DMF solution containing tetrabutyl ammonium perchlorate as a supporting electrolyte have been studied with cyclic voltammetry. These uranyl(VI) complexes were found to be quasi-reversibly reduced to U(V) species. The formal redox potentials (E
-diketonate = thenoyltrifluoroacetonate (ttfa), benzoyltrifluoroacetonate (btfa), and dibenzoylmethanate(dbm), trop = tropolonate, and sap = 2-salicylidenaminophenolate) complexes in DMF solution containing tetrabutyl ammonium perchlorate as a supporting electrolyte have been studied with cyclic voltammetry. These uranyl(VI) complexes were found to be quasi-reversibly reduced to U(V) species. The formal redox potentials (E ,
, 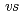 . ferrocene/ferrocenium) for U(VI)/U(V) couples were determined to be -1.18 V for UO
. ferrocene/ferrocenium) for U(VI)/U(V) couples were determined to be -1.18 V for UO (ttfa)
(ttfa) DMF, -1.18 V for UO
DMF, -1.18 V for UO (btfa)
(btfa) DMF, -1.46 V for UO
DMF, -1.46 V for UO (dbm)
(dbm) DMF, -1.46 V for UO
DMF, -1.46 V for UO (trop)
(trop) DMF, and -1.59 V for UO
DMF, and -1.59 V for UO (sap)(DMF)
(sap)(DMF) .
.
Arisaka, Makoto; Kimura, Takaumi; Nagaishi, Ryuji; Yoshida, Zenko
Journal of Alloys and Compounds, 408-412, p.1307 - 1311, 2006/02
Times Cited Count:6 Percentile:42.74(Chemistry, Physical)no abstracts in English
Shirai, Osamu*; Yamana, Hajimu*; Arai, Yasuo
Journal of Alloys and Compounds, 408-412, p.1267 - 1273, 2006/02
Times Cited Count:39 Percentile:84.47(Chemistry, Physical)no abstracts in English

 -edge of perpendicular magnetic Dy
-edge of perpendicular magnetic Dy Co
Co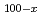 films
filmsAgui, Akane; Mizumaki, Masaichiro*; Asahi, Toru*; Sayama, Junichi*; Matsumoto, Koji*; Morikawa, Tsuyoshi*; Matsushita, Tomohiro*; Osaka, Tetsuya*; Miura, Yoshimasa*
Journal of Alloys and Compounds, 408-412, p.741 - 745, 2006/02
Times Cited Count:7 Percentile:46.38(Chemistry, Physical)no abstracts in English
Takahashi, Yoshio*; Murata, Miho*; Kimura, Takaumi
Journal of Alloys and Compounds, 408-412, p.1246 - 1251, 2006/02
Times Cited Count:24 Percentile:75.32(Chemistry, Physical)no abstracts in English
Abe, Hiroshi; Morimoto, Ryo*; Sato, Fumiatsu*; Azuma, Yorito*; Uchida, Hirohisa*
Journal of Alloys and Compounds, 408-412, p.348 - 350, 2006/02
Times Cited Count:10 Percentile:54.65(Chemistry, Physical)Mm(misch metal) based hydrogen storage alloys are applied to the negative electrode of the Ni-H battery and other hydrogen storage systems. In such pratical applications, various surface processes of hydrogen molecules often become rate contolling. Therefore, the improvement of the alloy surface is of great importance to promote the initial activation and hydriding rate. We have reported that the alkaline pretreatment of an alloy surfece exhibits a high durability against CO attack. Since low energy ion irradiation is quite useful for surface modification of materials, the hydriding proerties of a Mm is expected on electrochemical hydriding rate of the alloy. As a result, the ion irradiation Mm was found to induce a higher hydriding rate than that of the un-irradiation one.
Nankawa, Takuya; Suzuki, Yoshinori*; Ozaki, Takuo; Onuki, Toshihiko; Francis, A. J.*
Journal of Alloys and Compounds, 408-412, p.1329 - 1333, 2006/02
Times Cited Count:3 Percentile:28.65(Chemistry, Physical)We studied the biodegradation of Eu(III)-malic acid complexes by 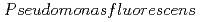 . Ten milimolar Malic acid was degraded in the absence and in the presence of Eu(III) of 0.05, 0.1, and 0.2 mM. The degradation rate of malic acid increased with decreasing the ratios of Eu(III) to malic acid. These results suggest that the toxicity of Eu(III) can be masked through its complexation with malic acid. The degradation of malic acid was followed by the production of unidentified metabolites which were associated with Eu(III). One of the unidentified organic acids was analysed to be pyruvic acid. Our findings suggest that metabolites can influence the environmental behavior of Eu(III) by biologically transformed through subsequent complexation with Eu(III).
. Ten milimolar Malic acid was degraded in the absence and in the presence of Eu(III) of 0.05, 0.1, and 0.2 mM. The degradation rate of malic acid increased with decreasing the ratios of Eu(III) to malic acid. These results suggest that the toxicity of Eu(III) can be masked through its complexation with malic acid. The degradation of malic acid was followed by the production of unidentified metabolites which were associated with Eu(III). One of the unidentified organic acids was analysed to be pyruvic acid. Our findings suggest that metabolites can influence the environmental behavior of Eu(III) by biologically transformed through subsequent complexation with Eu(III).
Ozaki, Takuo; Suzuki, Yoshinori*; Nankawa, Takuya; Yoshida, Takahiro; Onuki, Toshihiko; Kimura, Takaumi; Francis, A. J.*
Journal of Alloys and Compounds, 408-412, p.1334 - 1338, 2006/02
Times Cited Count:47 Percentile:87.21(Chemistry, Physical)We investigated the interactions of Eu(III) with the common soil bacterium Pseudomonas fluorescens and organic ligands, such as malic acid, citric acid, and a siderophore (DFO). Malic acid formed complexes with Eu(III), but degradation of malic acid was observed when the ratio of malic acid to Eu(III) was high. Citric acid formed a stoichiometric complex with Eu(III) that was not degraded by P. fluorescens. Adsorption of Eu(III) from the DFO complex occurred as a free ion dissociated from DFO and not as the Eu(III)-DFO complex. Time-resolved laser-induced fluorescence spectroscopy analysis showed that adsorption of Eu(III) on P. fluorescens was through a multidentate and predominantly inner-spherical coordination.
Fujii, Toshiyuki*; Asano, Hideki*; Kimura, Takaumi; Yamamoto, Takeshi*; Uehara, Akihiro*; Yamana, Hajimu*
Journal of Alloys and Compounds, 408-412, p.989 - 994, 2006/02
Times Cited Count:10 Percentile:54.65(Chemistry, Physical)no abstracts in English
 perovskite automotive catalyst having a self-regenerative function
perovskite automotive catalyst having a self-regenerative functionTanaka, Hirohisa*; Tan, Isao*; Uenishi, Mari*; Taniguchi, Masashi*; Kimura, Mareo*; Nishihata, Yasuo; Mizuki, Junichiro
Journal of Alloys and Compounds, 408-412, p.1071 - 1077, 2006/02
Times Cited Count:51 Percentile:88.55(Chemistry, Physical)no abstracts in English
Tateiwa, Naoyuki; Nakagawa, Akitoshi*; Fujio, Kazuhiko*; Kawae, Tatsuya*; Takeda, Kazuyoshi*
Journal of Alloys and Compounds, 408-412, p.244 - 247, 2006/02
Times Cited Count:2 Percentile:21.85(Chemistry, Physical)The rare earth metal praseodymium (Pr) transforms from the d-fcc crystal structure (Pr-III) to  -U one (Pr-IV) at 20 GPa with a large volume collapse (
-U one (Pr-IV) at 20 GPa with a large volume collapse (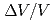 = 0.16), which is associated with the valence change of the Pr ion. The two 4f electrons in the Pr ion is supposed to be itinerant in the Pr-IV phase. In order to investigate the electronic state of the phase IV, we performed the high-pressure electrical resistance measurement using the diamond anvil cell up to 32 GPa. In the Pr-IV phase, the temperature dependence of the resistance shows an upward negative curvature, suggestting the narrow quasiparticle band of the 4f electrons near the Fermi energy. A new phase boundary is found at
= 0.16), which is associated with the valence change of the Pr ion. The two 4f electrons in the Pr ion is supposed to be itinerant in the Pr-IV phase. In order to investigate the electronic state of the phase IV, we performed the high-pressure electrical resistance measurement using the diamond anvil cell up to 32 GPa. In the Pr-IV phase, the temperature dependence of the resistance shows an upward negative curvature, suggestting the narrow quasiparticle band of the 4f electrons near the Fermi energy. A new phase boundary is found at 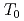 in the Pr-IV phase. From the temperature and magnetic field dependences of the resistance at 26 GPa, the ground state of the Pr-IV phase is suggested to be magnetic. Several possibilities for the origin of
in the Pr-IV phase. From the temperature and magnetic field dependences of the resistance at 26 GPa, the ground state of the Pr-IV phase is suggested to be magnetic. Several possibilities for the origin of 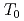 are discussed.
are discussed.
 Co
Co )
)
Mizumaki, Masaichiro*; Yoshii, Kenji; Watanabe, Yasuhiro*; Nanao, Susumu*
Journal of Alloys and Compounds, 408-412, p.737 - 740, 2006/02
Times Cited Count:3 Percentile:28.65(Chemistry, Physical)We have measured XAS and MCD spectra at Ce L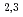 -edges in Ce(Fe
-edges in Ce(Fe Co
Co )
) . All the XAS spectra around the Ce-L
. All the XAS spectra around the Ce-L edge exhibit a double-peak structure that is the characteristic feature of the mixed-valence system. The ratio of the 4f
edge exhibit a double-peak structure that is the characteristic feature of the mixed-valence system. The ratio of the 4f peak intensity to the 4f
peak intensity to the 4f peak intensity in the Ce L
peak intensity in the Ce L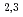 -edges increased as the composition of Co increased. But the MCD and XAS spectral shape changed slightly for Ce(Fe
-edges increased as the composition of Co increased. But the MCD and XAS spectral shape changed slightly for Ce(Fe Co
Co )
) . As the Co composition increased, MCD intensity decreased. Applying to Sum rules, we found that the orbital magnetic moment of Ce-5d electrons is very small but does exist.
. As the Co composition increased, MCD intensity decreased. Applying to Sum rules, we found that the orbital magnetic moment of Ce-5d electrons is very small but does exist.